
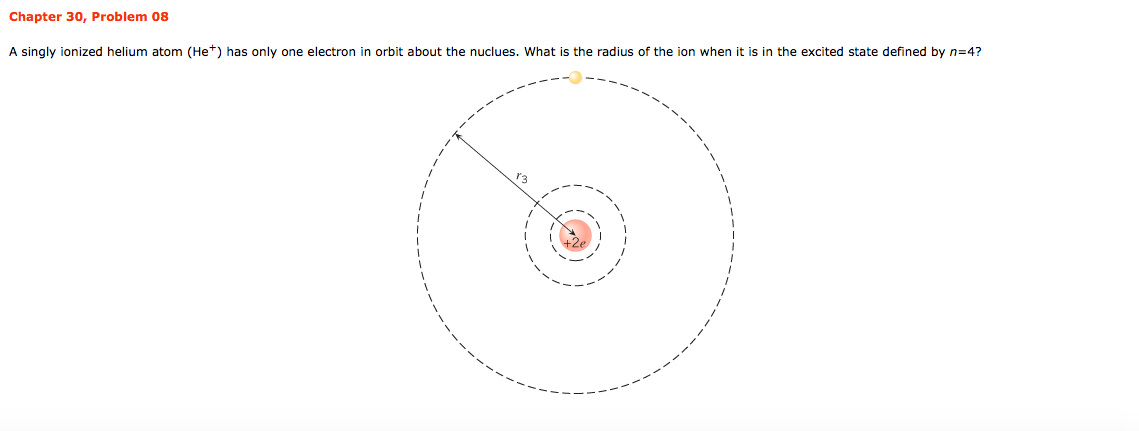
Singly ionized helium (He +) is a hydrogen-like atom.Determine the energy in eV required to raise a He + electron from the n = 1 to the n = 2 energy level. Explanation: Above is an image of the relatively quiet Sun made on May 18 in light emitted by ionized Helium atoms in the Solar chromosphere. Helium was first discovered in the Sun in 1868, its name fittingly derived from from the Greek word Helios, meaning Sun. Credit for the discovery goes to astronomer Joseph Lockyer.
Ionization of the most abundant elements, hydrogen and helium, is important for the equation of state. For these elements we have: ionization of hydrogen: χ = 13.54 eV, 2gi+1/gi = 1, first ionization of helium: χ = 24.48 eV, 2gi+1/gi = 4, second ionization of helium: χ = 54.17 eV, 2gi+1/gi = 1. Consider now pure, partly ionized hydrogen.
- Consider a hydrogen atom and a singly ionized helium atom. Which atom has the lower ground state energy? (a) Hydrogen (b) Helium (c) The ground state energy is the same for both.
- (Pickering Series) E.C. Pickering discovered ionized helium lines in the hot star Zeta Puppis in 1896, and mistaked it for a form of hydrogen. Later these lines are found in other hot emission line stars and Wolf-Rayet stars. Pickering was convinced that the lines were due to hydrogen under unknown temperature and pressure conditions.
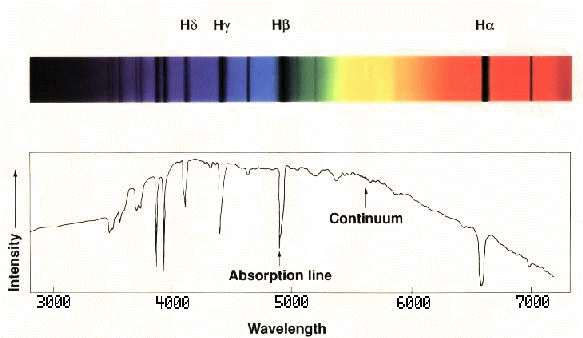
We may consider three principal types of spectra which appear when the light from an object is broken up into its component wavelengths or 'dispersed':
- a continuous spectrum or continuum; the emission of a thermal spectrum is one type of continuum.
- an absorption spectrum or sometimes an absorption-line spectrum.
- an emission spectrum or emission-line spectrum.
Honeywell usb devices driver download for windows. An absorption spectrum is produced when a continuum passes through 'cooler' gas. Photons of the appropriate energies are absorbed by the atoms in the gas.Although the photons may be re-emitted, they are effectively removed from the beam of light, resulting in a dark or absorption feature. The atmospheres of stars act as a cooler blanket around the hotter interior of a star so that typical stellar spectra are absorption spectra.
The systematic classification of stars in terms of absorption featuresand the understanding that suchspectral classification is essentially a sequence in atmospheric temperature was due to Annie J. Cannon at Harvard.
| Stellar Spectral Classification | |||||||
|---|---|---|---|---|---|---|---|
| Spectral Type | Atmospheric Temperature (K) | Hydrogen (Balmer) Features | Other Features | M/M | R/R | L/L | Main Sequence Lifetime |
| O | >33,000 K | weak | Ionized Helium (He+) features sometimes in emission Strong UV continuum | 20-60 | 9-15 | 90,000-800,000 | 10-1 Myr |
| B | 10,500-30,000 K | medium | Neutral He absorption | 3-18 | 3.0-8.4 | 95-52,000 | 400-11 Myr |
| A | 7,500-10,000 K | strong | H features maximum at A0 Some features of heavy elements, eg Ca+ | 2.0-3.0 | 1.7-2.7 | 8-55 | 3 Gyr - 440 Myr |
| F | 6,000-7,200 K | medium | 1.1-1.6 | 1.2-1.6 | 2.0-6.5 | 7-3 Gy | |
| G | 5,500-6,000 K | weak | Ca+ H&K, Na 'D' Sun is G2V | 0.9-1.05 | 0.85-1.1 | 0.66-1.5 | 15-8 Gy |
| K | 4,000-5,250 K | v. weak | Ca+, Fe, Strong molecules, eg CH, CN | 0.6-0.8 | 0.65-0.80 | 0.10-0.42 | 17 Gy |
| M | 2,600-3,850 K | v. weak | Molecules, eg TiO Very red continuum | 0.1-0.5 | 0.17-0.63 | 0.001-0.08 | 56 Gy |
| L | 1300-2,500K | Metal hydride molecular bands (CrH & FeH); neutral metallines | ~ 0.08 | ||||
| T | < 1300 | Methane bands | < 0.08 | ||||
'R', 'N', 'S' stars are cool stars with particular types of molecular bands.
'L' stars are possibly not truly stars at all, in the sense that they may not have nuclear reactions at their cores.
Click on the spectral type above to see spectra of the appropriate type or click here to see Spectral Types O - Gand here for Spectral Types G - M
Click here for a selection of Spectral Sequencemnemonics
The Stellar Spectral Sequence as a Temperature Sequence
The temperature of the stellar photosphere determines the rate and severity of collisions between molecules, atoms and ions which in turn determines:
- the molecular equilibrium - if the star is too hot, fragile molecular bonds will be broken apart. Most molecules such as TiO are seen only in spectra of the coolest stars ( T = 3000 -- 4000K). Strongmolecules such as CH and CN can be seen in somewhat hotter stars like the sun.
- the ionization equilibrium - the hotter the temperature, the higher theionization state of the atoms in the stellar atmosphere will be. Atoms are ionized (or partially ionized) when they lose or gain anelectron. In cool stars most atoms will be neutral. At higher Temperature, easily ionized atoms such as Na, Ca, etc, will be ionized; above T = 10,000K hydrogen becomes ionized and above about 15,000K helium becomes ionized.
- the number of atoms in excited states. At low T, almost no H atoms are in the n=2 orbit, capable of absorbing 'Balmer' photons, but as T increases the n=2 population increases and we see the hydrogen features, reaching a maximum in stars with T = 10,000.
Among the other things that we may determine from the absorption spectrum are: density, chemical composition, magnetic field strength, and radial velocity. These are all secondary effects compared to temperature.
Jim Kaler's StarsWebsite dedicated to showing that not all stars are the same.The SunThermal Radiation Physics 7 Lectures Physics 7 Home
Conducted by Gene Smith, CASS/UCSD.Comments? You may send email to hsmith@ucsd.edu
Prof. H. E. (Gene) Smith
CASS 0424 UCSD
9500 Gilman Drive
La Jolla, CA 92093-0424
Last updated: 28 Jan 2000
(Pickering Series)
E.C. Pickering discovered ionized helium lines in the hot star Zeta Puppis in 1896, and mistaked it for a form of hydrogen. Later these lines are found in other hot emission line stars and Wolf-Rayet stars. Pickering was convinced that the lines were due to hydrogen under unknown temperature and pressure conditions. What was then called the 'additional hydrogen lines' or the Pickering series could be fitted to the Balmer formula, provided half integral quantum numbers were allowed. Lockyer called the spectrum of ionized heliumDouble Ionized Helium
'proto-hydrogen'| He II: Energy levels of singly ionized Helium. Pickering series of lines is marked in red. Photoionization thresholds are marked in dashed lines (Series Limits). The five transitions to the ground (n=1) level are not labeled. |
The transitions between the singly ionized helium energy levels corresponding to the Pickering series are 4-5 (10124 Å), 4-7 (5412 Å) and 4-9 (4522 Å) (red in above diagram). Note that the 4-6 (6560 Å) and 4-8 (4859 Å) (green in diagram) transitions were originally not included in this series because they coincided with the hydrogen series of lines and were thus obscured (except in hydrogen deficient stars). Although the 3-4 (4686 Å) (blue in diagram) transition also belongs to ionized helium and often occurs in these hot stars, it did not belong to the pickering series because it has a lower landing level quantum number (n=3 instead of n=4).
In 1912, Pickering still believed it was hydrogen, even when one of Lockyer's pupils, Alfred Fowler showed that these lines are produced in a laboratory mixture of hydrogen and helium. This unusual oversight could partially be attributed to the fact that Fowler still subscribed to Pickering's belief that it was hydrogen. (Just another example that belief is stronger than reason !).

At the turn of the century the physicist Max Planck discovered energy quantization in his theoretical analysis of black body radiation. Albert Einstein later established that these energy quanta are photons or particles of light. Then came Rutherford's model of the atom made up of small massive positively charged nucleus surrounded by a relatively large volume occupied by the negatively charged electrons.
The physicist Niels Bohr, founding father of quantum mechanics, synthesized all these separate facts into a coherent picture of the atom in which the electron orbits were quantized into stationary states and exchange energy with the electromagnetic field in discrete energy packets called photons. Drivers pega pc. His theory predicted the spectrum of singly ionized Helium which corresponded exactly to the Pickering line series. The helium nuclei has twice the charge of the hydrogen nuclei, this gave rise to Pickering's spurious half-integral quantum numbers.
COMMENTARY
This was not the last time that spectral lines at unusual wavelengths were mistaken for hydrogen in strange hypothetical environments; Maarten Schmidt (1963) has also mistaken the emission lines in the spectra of quasars with hydrogen, and this historical error is still not generally recognized.Ionization Energy Helium
REFERENCES
- Pickering,E.C.: 1896, Astrophys.J., 4, 369.
- Pickering,E.C.: 1897, Harvard Circ.16.
- Pickering,E.C.: 1897, Harvard Circ.18.
- Lockyer,J.N.: 1900, Inorganic Evolution, Macmillan and Co. (London).
- Pickering,E.C.: 1901, Harvard Circ.55.
- Fowler,A.: 1912, Mon.Not.R.Astron.Soc.73, 62.
- Planck,M.: 1901, Wiedemanns Ann. der Physik, 4, 553.
- Einstein,A.: 1905, Ann. der Physik, 17, 132.
- Rutherford,E.: 1911, Phil.Mag.21 (Ser 6), 669.
- Bohr,N.: 1913, Phil.Mag., 26 (Ser 6), 1.
Ionized Helium
Next historical event